This is the second article of a series on “The New Epigenetics”. The epigenetic perspective in biology and psychology provides a solution to the clunky, age-old nature-nurture issue. It says that the old view that pits nature against nurture (genes against environment) as rival explanations for how development happens is flawed. Genes and environments are partners in development; they can’t work without each other. This has radical implications for how we understand biology, development, and human nature. Because different political ideologies are build largely around different conceptions of human nature, it has deep implications for political life as well.
We tend to think of genes as blueprints for our bodies. Some even think that genes are blueprints for how we think, feel and act! We tend to think that But the situation is far more complex than that. We are not claiming that genes do not play a central role in building our bodies. They do. Genes play an essential role in all aspects of an organism’s functioning. The point here is that genes cannot and do not work alone to create developmental outcomes.
Development is Open-Ended – Even the Development of our Bodies
Anatomical structures are not the simple results of the unfolding of a genetic plan. Even a few moments of reflection show how this must be the case. Imagine we have different sets of seeds. Imagine that the seeds in different sets have different genes, but that the seeds in each individual set have the same genes. Imagine that we plant these seeds at different levels of elevation on a mountain? What will happen?
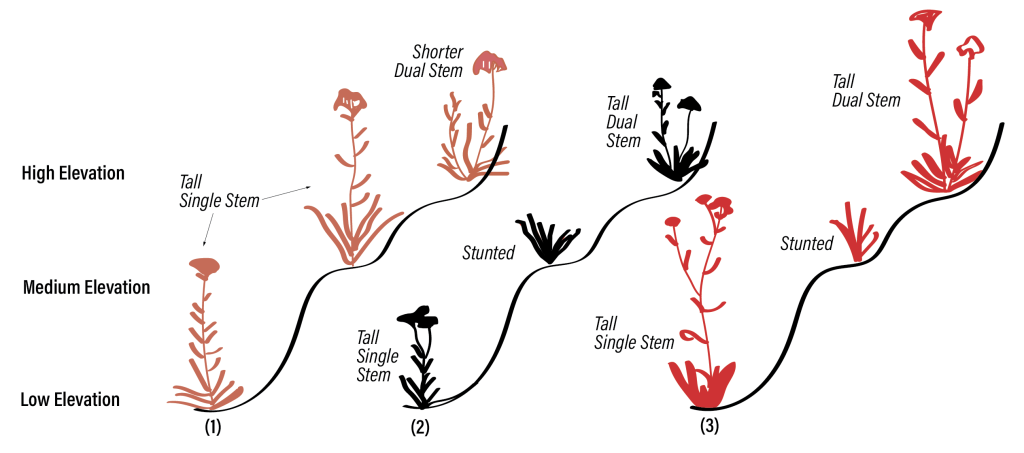
Figure 1. Plants with the same genes develop differently in different environments. Plants with different genes develop differently in the same environment.
The answer is shown in Figure 1. The figure shows was happens to three different strains of plants when the grow at different elevations. The different strands – (1), (2) and (3) – have different genes. However, within each strain, each of the three plants shown have the same genes. As shown in the Figure, the environment in which the seeds are planted matters. The physical structure of each strain of plant is different at different elevations. In the first strain (1), both low and medium elevations produce tall plants with a single stem. At the highest elevation, the plant is shorter, and sprouts two stems. These plants share the same genes – what differs here is the environment in which they are planted. We can imagine that seeds planted at the highest evolution develop in suboptimal conditions – less oxygen, colder temperatures, less fertile ground, and so forth. We can imagine that this is why the plants at the highest level are shorter than those at lower levels.
But this is not always the case! We can’t tell beforehand what the outcome of development will be! Strains (2) and (3) — whose genes differ both from each other and Strain (1) — show different outcomes of development in different elevations. For both strains, seeds planted at middle elevations produce the smallest plants without flowering stems. For both strains, plants at the lowest and highest elevations are equally tall! Plants at lower levels have but one stem, while plants at the highest level have two.
It follows that there are many outcomes to development depending on the particularities of how genes interact with their local environments. We can’t predict for certain beforehand how a plant will grow. If we want to know how development happens, we have to actually look and see. We have to look at how individual organisms interact with particular environments at different points in time.
Examples of Open-Ended Development in Plants, Animals and Humans
There are many additional examples of this process. Table 1 contains a series of examples of how the environmental conditions of development modify the development of physical anatomy in different species. As shown in the table, the onset of budding in plants is modified by changes in the timing of the seasons – including climate change. Fruit flies exposed to ether in the embryonic stage develop a second pair of wings. There is not genetic plan that determines which ant or bee becomes the Queen; the course of development depends on whether the bee develops within the physical environment of the “royal jelly; the development of Queen bees depends on social hierarchies in the colony. It is well known that many varieties of fish change sex as a product of environmental changes (shifts in the availability of males versus females; shifts in social hierarchies). Modifications of nutrition modify the development of metabolism in rats and other species.
Table 1
The Epigenetic Development Anatomical Structures in Different Species
| Species | Structure/Process | Environmental Condition | Outcome |
| Plants | Onset of budding | Timing of seasons; climate change | Variation in time of flowering1 |
| Fruit Flies | Wings | Ether introduced in embryo | Second pair of wings2 |
| Ants and Bees | Queen versus Worker Bee | “Royal Jelly”
Social conditions |
Development into queen or worker bee (same genome) 3 |
| Fish | Sexual Anatomy | Stressors; changes in status hierarchies | Change of sex4 |
| Rats | Liver | · Decreased nutrition.
· Stress |
Modification of glucose/fat metablism5 |
| Humans | Brain activity | Maternal use of cigarettes or cannabis | Alternations of genetic activity in brain ” risk of later psychiatric disorders6 |
| Humans and other animals | Internal and external sexual anatomy | Presence or absence of testosterone, estrogen and other enzymes | Development of intersex reproductive anatomy7 |
| Humans | Propensity toward physical diseases | Low socio-economic status | Faster aging of immune cells ” increase probability of age-related disease8 |
1De Jong & Leyser (2011); 2Ho (1983), 3Alhosin, 2022; Villalta et al.., (2016); 4Ortega-Recalde et al., (2020); Heo et al., 2018; McGowan et al., 2008; 6Morris et al., (2011); 8Austin et al., (2018)
In humans, the development of sexual anatomy (penis and testicles in the male; ovaries and uterus in the female, etc.) depends on the presence or absence of hormones (testosterone, estrogen, etc.) and enzymes (H-Y antigens) in the biological environment of the developing organs. Changes in the presence and timing of sex hormones can lead to the development of opposite sex anatomy. This is how many intersex forms of sexual anatomy arise. Maternal use of tobacco or cannabis is associated with modified brain structures in offspring (and with later psychiatric diagnoses). Processes associated with low socioeconomic status during development are associated with faster aging of immune cells and with a suite of physical diseases.
Examples of Epigenetic Development of Behavior and Experience
If physical anatomy is not under genetic control, there is certainly no reason to believe that behavior and experience – processes that are more complex than anatomy – are products of fixed genetic plans. Table 2 contains examples of how changes in environment and rearing conditions result in changes in behaviors that, in earlier times, were often thought to be under genetic control. The song of the cricket is influenced by temperature and length of day. Rats become more docile when they are handled gently after birth. In rodents, males are less likely to kill offspring after rather than before becoming fathers. Cats raised with mice are less likely to kill them. Mallard ducks must hear their own or their mother’s call in utero if they are to recognize their mother’s call after birth. Birds are more likely to nest in trees and other locations above the ground in the presence of prey who live on land (e.g., snakes). The timing of parturition (pregnancy) is modified by changes in environment, including availability of food, temperature, and other aspects of climate. The position of opposite sex twins in utero is associated with the development of behavior typical of the opposite sex in later years. While some children exhibit biological biases toward aggressive and callous behavior toward others, long term aggression is modified by firm yet sensitive parenting.
And this is but a short list. The list goes on.
Table 2
The Epigenetic Development of Behavior and Experience in Different Species
| Species | Behavior/
Experiential State |
Environmental Condition | Outcome |
| Cricket | Pulsating (“song”) | Temperature and day length | Changes in form of “song” 1 |
| Rats | Emotionality | Postnatal handling | Diminished stress2 |
| Rodents | Male behavior toward offspring | Before versus after fathering pups | Diminished killing of offspring3. |
| Ducks | Responding to material call | Prevent from hearing the duck’s own or the duck’s mother’s call | Failure to recognize material call4 |
| Cats | Mice killing | Raise cats with mice | No mice killing5 |
| Warblers | Nest Building | Presence of land-dwelling prey | Build nests in trees rather than on land6 |
| Bighorn Sheep | Timing of parturition (birth) | Shift in temperature; climate change; availability of food | Shifting time of parturition7 |
| Humans | Sex-typical behavior | Position of female fetus in relation to male fraternal twin in the womb | Females gestated with males show increased opposite sex behaviors8 |
| Humans | Aggressive behavior | Early exposure to adversity (abuse, harsh parenting, etc.) | Stable long-term aggression9 |
1Beckers (2020); 2Dennenberg (1964); 3Inada et al., (2023); 4Gottlieb (2007), 5Kuo (1930); 6Szymkowiak & Thomson (2019); 7Renaud et al., (2019); 8Kawata (2013); 9Chistiakov et al., (2019); Pishva et al, (2023)
The research findings showing the role of epigenetic processes in the physical and psychological processes is overwhelming and growing. We can no longer think about genes and environments as separable processes in the development of individuals. If we change genes, we change outcomes; if we change environments, we change outcomes. Developmental outcomes are products of relations between genes and multiply nested environments. No one cause acts alone.
References
Abel, T., & Poplawski, S. (2022). Epigenetic advances in clinical neuroscience. Dialogues in clinical neuroscience.
Alhosin M. (2023). Epigenetics Mechanisms of Honeybees: Secrets of Royal Jelly. Epigenetic Insights. Nov 29, 16.
Atlasi, Y., & Stunnenberg, H. G. (2017). The interplay of epigenetic marks during stem cell differentiation and development. Nature Reviews Genetics, 18(11), 643-658.
Austin, M. K., Chen, E., Ross, K. M., McEwen, L. M., Maclsaac, J. L., Kobor, M. S., & Miller, G. E. (2018). Early-life socioeconomic disadvantage, not current, predicts accelerated epigenetic aging of monocytes. Psychoneuroendocrinology, 97, 131–134. https://doi-org.proxy3.noblenet.org/10.1016/j.psyneuen.2018.07.007
Beckers, O. M. (2020). Phenotypic plasticity related to temperature induces song variation in the field cricket Gryllus rubens. Ethology, 126(8), 781–790. https://doi-org.proxy3.noblenet.org/10.1111/eth.13035
Brunton, P. J. (2010). Resetting the dynamic range of hypothalamic‐pituitary‐adrenal axis stress responses through pregnancy. Journal of neuroendocrinology, 22(11), 1198-1213.
Cañas, J. A., Núñez, R., Cruz-Amaya, A., Gómez, F., Torres, M. J., Palomares, F., & Mayorga, C. (2021). Epigenetics in food allergy and immunomodulation. Nutrients, 13(12), 4345.
Castillo-Fernandez, J. E., Spector, T. D., & Bell, J. T. (2014). Epigenetics of discordant monozygotic twins: implications for disease. Genome medicine, 6(7), 1-16.
Cecil, C. A., & Nigg, J. T. (2022). Epigenetics and ADHD: Reflections on current knowledge, research priorities and translational potential. Molecular diagnosis & therapy, 26(6), 581-606.
Chistiakov, D. A., & Chekhonin, V. P. (2019). Early-life adversity-induced long-term epigenetic programming associated with early onset of chronic physical aggression: Studies in humans and animals. The World Journal of Biological Psychiatry : The Official Journal of the World Federation of Societies of Biological Psychiatry, 20(4), 258–277. https://doi-org.proxy3.noblenet.org/10.1080/15622975.2017.1322714
Denenberg V.H.. (1964). Critical periods, stimulus input, and emotional reactivity: A theory of infantile stimulation. Psychological Review, 71, 335-351.
de Jong M, Leyser O. Developmental plasticity in plants. Cold Spring Harb Symp Quant Biol. 2012;77: 63–73. pmid:23250989
Gottlieb, G. (2007). Social induction of malleability in ducklings. European Journal of Developmental Science, 1(3), 212–226.
Heijmans, B. T., Tobi, E. W., Stein, A. D., Putter, H., Blauw, G. J., Susser, E. S., … & Lumey, L. H. (2008). Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proceedings of the National Academy of Sciences, 105(44), 17046-17049.
Heo, H. J., Tozour, J. N., Delahaye, F., Zhao, Y., Cui, L., Barzilai, N., & Einstein, F. H. (2016). Advanced aging phenotype is revealed by epigenetic modifications in rat liver after in utero malnutrition. Aging Cell, 15(5), 964–972. https://doi-org.proxy3.noblenet.org/10.1111/acel.12505
Ho M.W, Tucker C, Keeley D., & Saunders P.T. (1983). Effects of successive generations of ether treatment on penetrance and expression of the Bithorax phenocopy in Drosophila melanagaster. Journal of Experimental Zoology, 225, 357–368.
Inada, K., & Miyamichi, K. (2023). Association between parental behaviors and structural plasticity in the brain of male rodents. Neuroscience Research, 196, 1–10. https://doi-org.proxy3.noblenet.org/10.1016/j.neures.2023.06.007
Kawata, M. (2013). Nurture: effects of intrauterine position on behaviour. Journal of Neuroendocrinology, 25(4), 422–423. https://doi-org.proxy3.noblenet.org/10.1111/jne.12026
Konkel, L. (2016). Lasting impact of an ephemeral organ: the role of the placenta in fetal programming.
Kuo, Z. Y. (1930). The genesis of the cat’s responses to the rat. Journal of Comparative Psychology, 11(1), 1–36. https://doi-org.proxy3.noblenet.org/10.1037/h0075723
Lintas, C. (2019). Linking genetics to epigenetics: The role of folate and folate‐related pathways in neurodevelopmental disorders. Clinical Genetics, 95(2), 241-252.
Matosin, N., Cruceanu, C., & Binder, E. B. (2017). Preclinical and clinical evidence of DNA methylation changes in response to trauma and chronic stress. Chronic Stress, 1, 2470547017710764.
McGowan, P. O., Meaney, M. J., & Szyf, M. (2008). Diet and the epigenetic (re)programming of phenotypic differences in behavior. Brain Research, 1237, 12–24. https://doi-org.proxy3.noblenet.org/10.1016/j.brainres.2008.07.074
Mehta, D., Bruenig, D., Pierce, J., Sathyanarayanan, A., Stringfellow, R., Miller, O., … & Shakespeare-Finch, J. (2022). Recalibrating the epigenetic clock after exposure to trauma: The role of risk and protective psychosocial factors. Journal of psychiatric research, 149, 374-381.
Morris, C. V., DiNieri, J. A., Szutorisz, H., & Hurd, Y. L. (2011). Molecular mechanisms of maternal cannabis and cigarette use on human neurodevelopment. European Journal of Neuroscience, 34(10), 1574–1583. https://doi-org.proxy3.noblenet.org/10.1111/j.1460-9568.2011.07884.x
Nestler, E. J. (2014). Epigenetic mechanisms of depression. JAMA psychiatry, 71(4), 454-456.
Ortega-Recalde, O., Goikoetxea, A., Hore T. A., Todd, E.V., Gemmell, N. J. (2020). The Genetics and Epigenetics of Sex Change in Fish. Annual Review of Animal Bioscience, 47-69. doi: 10.1146/annurev-animal-021419-083634.
Osborne, A. (2017). The role of epigenetics in human evolution. Bioscience Horizons: The International Journal of Student Research, 10.
Pal, S., & Tyler, J. K. (2016). Epigenetics and aging. Science advances, 2(7), e1600584.
Pishva, E., van den Hove, D. L. A., Laroche, V., Lvovs, A., Roy, A., Ortega, G., Burrage, J., Veidebaum, T., Kanarik, M., Mill, J., Lesch, K., & Harro, J. (2023). Genome‐wide DNA methylation analysis of aggressive behaviour: A longitudinal population‐based study. Journal of Child Psychology and Psychiatry, 64(7), 998–1006. https://doi-org.proxy3.noblenet.org/10.1111/jcpp.13782
Renaud, L.-A., Pigeon, G., Festa-Bianchet, M., & Pelletier, F. (2019). Phenotypic plasticity in bighorn sheep reproductive phenology: From individual to population. Behavioral Ecology and Sociobiology, 73. https://doi-org.proxy3.noblenet.org/10.1007/s00265-019-2656-1
Smith, S. M., & Vale, W. W. (2006). The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues in clinical neuroscience, 8(4), 383–395. https://doi.org/10.31887/DCNS.2006.8.4/ssmith
Szymkowiak, J., & Thomson, R.L. (2019). Nest predator avoidance during habitat selection of a songbird varies with mast peaks and troughs. Behavioral Ecology and Sociobiology, 73, 91. https://doi.org/10.1007/s00265-019-2702-z
Thomson, J. P., Ottaviano, R., Buesen, R., Moggs, J. G., Schwarz, M., & Meehan, R. R. (2017). Defining baseline epigenetic landscapes in the rat liver. Epigenomics, 9(12), 1503–1527. https://doi-org.proxy3.noblenet.org/10.2217/epi-2017-0029
Tobi, E. W. (2013). Epigenetic differences after prenatal adversity: the Dutch hunger winter (Doctoral dissertation, Leiden University).
Villalta, I., Blight, O., Angulo, E., Cerdá, X., & Boulay, R. (2016). Early developmental processes limit socially mediated phenotypic plasticity in an ant. Behavioral Ecology and Sociobiology, 70(2), 285–291. https://doi-org.proxy3.noblenet.org/10.1007/s00265-015-2052-4
Zhang, T. Y., Labonté, B., Wen, X. L., Turecki, G., & Meaney, M. J. (2013). Epigenetic mechanisms for the early environmental regulation of hippocampal glucocorticoid receptor gene expression in rodents and humans. Neuropsychopharmacology, 38(1), 111-123.


If you like what we are doing, please support us in any way that you can.